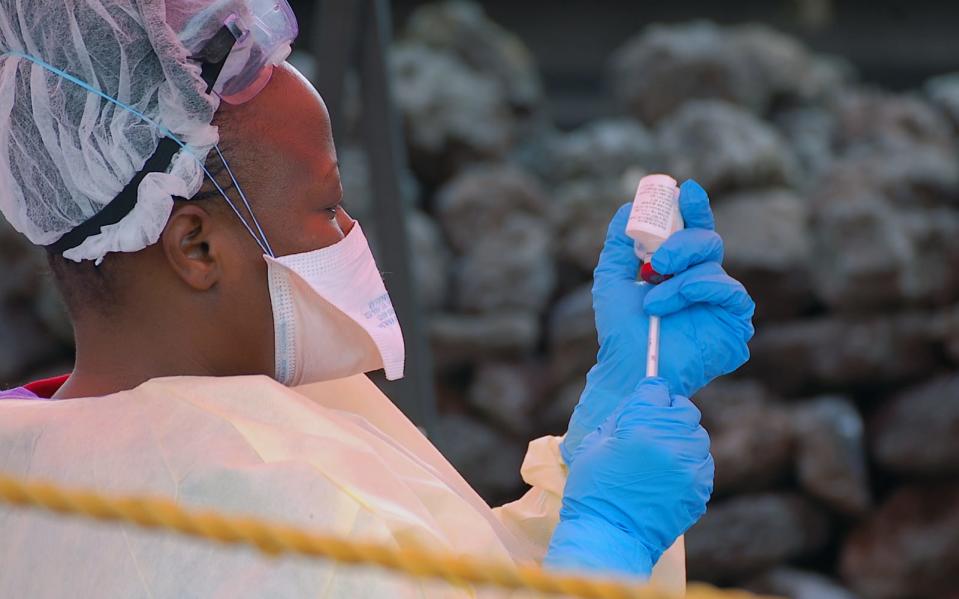
The EU just approved a vaccine to prevent Ebola
Ervebo is the first human Ebola vaccine to receive EU approval.
Today, the European Union granted an Ebola vaccine "conditional marketing authorization." The vaccine, developed by the pharmaceutical giant Merck, is known as Ervebo. It's the first human Ebola vaccine to be approved by the EU.
Ervebo was first engineered by the Public Health Agency of Canada and the US Army more than a decade ago. In 2014, following the Ebola outbreak in West Africa, Merck acquired the rights to develop the vaccine.
The "conditional marketing authorization" is a type of approval reserved for medicine that addresses unmet medical needs. It allows the medicine to advance with less comprehensive data than normally required if the benefits outweigh the risks. Preliminary Ervebo tests have been promising, with the vaccine proving to be 100 percent effective during trials in Guinea, and as part of the clinical development, Merck has donated more than 250,000 doses to the World Health Organization (WHO).
Merck plans to begin manufacturing Ervebo in Germany beginning in the third quarter of 2020. The vaccine is also under priority review with the US Food and Drug Administration (FDA).
According to the European Commission the Ebola outbreak in West Africa that began in 2014 killed more than 11,000 people. The current outbreak in the Democratic Republic of Congo (DRC) has shown a 67 percent fatality rate, and more than 3,000 people have been infected with the virus. The WHO declared Ebola a public health emergency of international concern in July, and that warning remains in effect.